Committed to improving your life during the battle against SLE.
Learn about a new clinical trial enrolling eligible participants with systemic lupus erythematosus (SLE) who have moderate to severe SLE.
About the
REMESLE-1 Study
The REMESLE-1 Study is a global Phase 3 clinical trial studying the investigational medication, telitacicept, to determine if it can help patients who have moderately to severely active SLE. The aim of the study is to evaluate the safety, efficacy and optimal dose level of telitacicept.
About the Study
Medication
The study medication, telitacicept, is a novel Fc fusion protein targeting BLyS and APRIL, two cell-signaling ligands critical for B-lymphocyte development, which play a role in antibody production in autoimmune diseases. Telitacicept acts to potentially inhibit the activity of these ligands to reduce the autoimmune symptoms commonly experienced in SLE.
Has the Study Medication been
used by patients before?
Telitacicept is approved in China to treat patients with moderately to severely active SLE. Since 2011, telitacicept has been studied or is being studied in over 16 clinical trials conducted in China for 7 autoimmune disease indications: SLE, rheumatoid arthritis (RA), immunoglobulin A nephropathy (IgAN), neuromyelitis optica spectrum disorders (NMOSD), myasthenia gravis (MG), primary Sjogren's Syndrome (pSS) and multiple sclerosis (MS).
Who is
REMESLE-1 for?
You may consider participating in this study if you are on standard lupus medication but continue to experience moderate to severely active SLE. Your participation will help investigators learn more about SLE and the investigational medicine, and the trial results may help other patients with lupus in the future. It is also possible that you may see some improvement in your lupus symptoms during your participation in this trial.
What can you expect
during the study?
You may consider participating in this study if you are on standard lupus medication but continue to experience moderate to severely active SLE. Your participation will help investigators learn more about SLE and the investigational medicine, and the trial results may help other patients with lupus in the future. It is also possible that you may see some improvement in your lupus symptoms during your participation in this trial.
Who is eligible to
participate in the
REMESLE-1 Study?
To be considered eligible for participation, you must meet the following requirements:
- You are between ages 18 to 70 years old
- You have had a diagnosis of SLE for at least 6 months prior to the screening visit
- You have moderate to severely active SLE
- You currently receive at least one standard of care SLE medication
In addition, participants must meet other study criteria in order to enroll in the study. For full eligibility criteria, please visit clinicaltrials.gov or reach out to a study site to see if you qualify.
Does it cost anything
to participate?
All study assessments, investigational study drug, and procedures will be provided at no cost to participants.
The REMESLE-1 Trial is
Enrolling Now
The RemeSLE-1 Study is currently enrolling in study sites across the world, including the United States, European Union, South America and in the Asia-Pacific Region including Australia. If you are interested, please contact the study site closest to you to learn if you are eligible to participate.
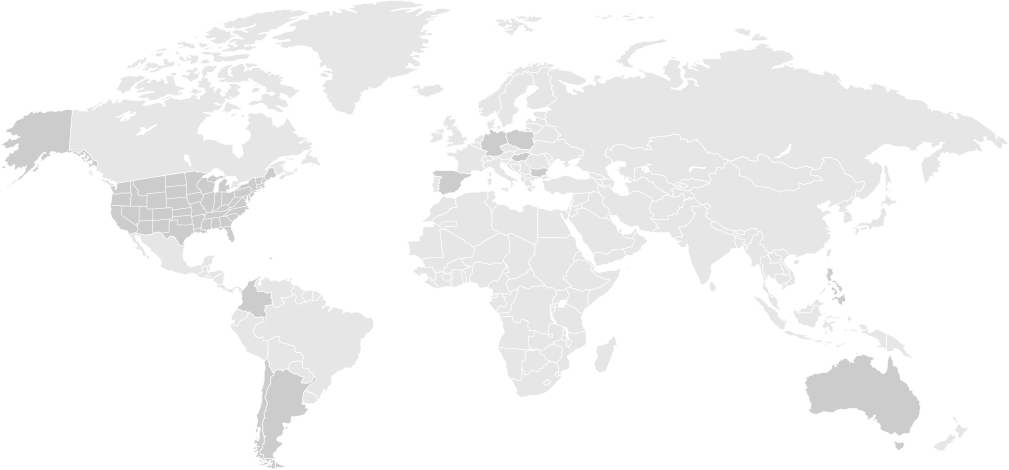 Alabama
Argentina
Australia
Bulgaria
California
Chile
China
Colombia
Florida
Germany
Hungary
Illinois
Michigan
Minnesota
North Carolina
Philippines
Poland
Puerto Rico
Spain
Texas
Alabama
Argentina
Australia
Bulgaria
California
Chile
China
Colombia
Florida
Germany
Hungary
Illinois
Michigan
Minnesota
North Carolina
Philippines
Poland
Puerto Rico
Spain
Texas
Click on the country for more information Coming Soon Recruiting